The nucleoside transport proteins, NupC and NupG, from Escherichia coli: specific structural motifs necessary for the binding of ligands†
Abstract
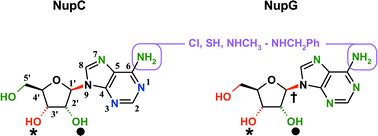
A series of 46 natural nucleosides and analogues (mainly adenosine-based) were tested as inhibitors of [U-14C]uridine uptake by the concentrative, H+-linked nucleoside transport proteins NupC and NupG from Escherichia coli. The two evolutionarily unrelated transporters showed similar but distinct patterns of inhibition, revealing differing selectivities for the different nucleosides and their analogues. Binding of nucleosides to NupG required the presence of hydroxyl groups at each of the C-3′ and C-5′ positions of ribose, while binding to NupC required only the C-3′ hydroxyl substituent. The greater importance of the ribose moiety for binding to NupG is consistent with the evolutionary relationship between this protein and the oligosaccharide: H+ symporter (OHS) subfamily of the major facilitator superfamily (MFS) of transporters. For both proteins the natural α-configuration at C-3′ and the natural β-configuration at C-1′ was mandatory for ligand binding. N-7 in the imidazole ring of adenosine and the amino group at C-6 were found not to be important for binding and both transporters showed flexibility for substitution at C-6/N6; one or both of N-1 and N-3 were important for adenosine analogue binding to NupC but significantly less so for binding to NupG. From the different effects of 8-bromoadenosine on the two transporters it appears that adenosine selectively binds to NupC in an anti- rather than a syn-conformation, whereas NupG is less prescriptive. The pattern of inhibition of NupC by differing nucleoside analogues confirmed the functional relationship of the bacterial transporter to members of the human concentrative nucleoside transporter (CNT) family and reaffirmed the use of the bacterial protein as an experimental model for these physiologically and clinically important mammalian proteins. The specificity data for NupG have been used to develop a homology model of the protein's binding site, based on the X-ray crystallographic structure of the disaccharide transporter LacY from E. coli. We have also developed an efficient general protocol for the synthesis of adenosine and three of its analogues, which is illustrated by the synthesis of [1′-13C]adenosine.

 Please wait while we load your content...
Please wait while we load your content...