Effect of aqueous acetic, oxalic and carbonic acids on the adsorption of uranium(vi) onto α-alumina
Abstract
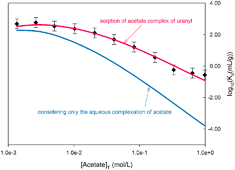
The prediction of the migration for radionuclides in geologic media requires a quantitative knowledge of retardation phenomena. For this purpose, the sorption of U(VI) onto a model mineral-–α-alumina—is studied here, including the effects of groundwater chemistry: pH and concentrations of small organic ligands (acetate, oxalate and carbonate anions). This work presents experimental evidence for the synergic sorption of uranium(VI) and the small organic ligands, namely sorption of cationic complexes onto alumina. Conversely, since its neutral and anionic complexes were not sorbed, U(VI) cation could also be desorbed as a result of the formation of neutral or anionic complexes in the aqueous phase. By using the ion-exchange theory, and a corresponding restricted set of parameters—exchange capacities and thermodynamic equilibrium constants—the whole set of sorption experiments of U(VI) cationic species onto the α-alumina was modelled under various chemical conditions.

 Please wait while we load your content...
Please wait while we load your content...