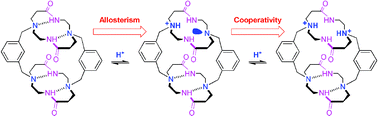
Synthesis, characterization and X-ray crystal structures of cyclam derivatives. 7. Hydrogen-bond induced allosteric effects and protonation cooperativity in a macrotricyclic bisdioxocyclam receptor†‡
Abstract
The unprecedented cooperative protonation properties displayed by a barrel-shaped macrotricyclic
 Please wait while we load your content...
Please wait while we load your content...