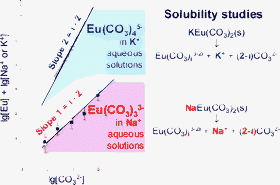
Within the framework of environmental studies regarding radioactive waste management, the speciation of M3+ f-elements in concentrated carbonate solutions was investigated. Solubility measurements of NaEu(CO3)2·5H2O(s) were performed at 23 ± 1 °C under fixed [Na+], by using NaClO4. In 3 mol l−1 Na+ aqueous solutions, the limiting complex Eu(CO3)33− was identified in the range 0.01 < [CO32−] < 2 mol l−1; experimental solubilities were modelled with the equilibrium: NaEu(CO3)2·5H2O(s) + CO32−
⇌ Na+ + Eu(CO3)33− + 5H2O, and the logarithm of the corresponding equilibrium constant was found to be logKs,3 = −4.2 ± 0.2. To interpret the measurements at different [Na+], the activity coefficients of the species were calculated with the SIT formula. Time-resolved laser-induced fluorescence spectroscopy was used to verify the presence of a single complex of Eu(III) in aqueous solutions. From fluorescence lifetime measurements in 1 mol l−1 Na2CO3 solutions in H2O–D2O mixtures, it was determined that about two water molecules remained in the first coordination sphere of Eu(CO3)33−. From a critical review of literature data and sensitivity analysis, it was concluded that solubility results should be more reliable than solvent extraction and spectroscopic results in determining the stoichiometry of limiting carbonate complexes. Tricarbonate complexes of most M3+ f-elements appeared to be the limiting stable species in Na+ aqueous solutions, except for Ce(III), for which a tetracarbonate complex was indeed observed. Interestingly, supporting electrolytes based on K+ can favour tetracarbonate complexes at high ionic strength. Available values of thermodynamic constants related to various ionic media are compared on the basis of the SIT formula.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...