A solid-state structural and theoretical study on the 1 ∶ 1 addition compounds of thioethers with dihalogens and interhalogens I–X (X = I, Br, Cl)†
Abstract
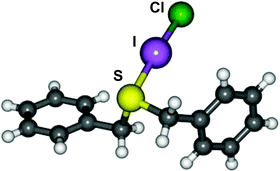
Two new thioether interhalogen adducts, (PhCH2)2S·ICl (1) and (PhCH2)2S·IBr (2) have been prepared and characterised in the solid-state, the prior being the first crystallographically characterised example of a thioether ICl charge transfer complex. Theoretical calculations have been carried out on a related series of thioether IX adducts (X = F, Cl, Br, I) and reveal the computed geometries for these systems, in particular the S–I and I–X distances, to vary significantly based on the choice of basis set and type of density functional hybridisation model employed. In order to model these electron rich adducts accurately we demonstrate the importance of using high quality basis sets and density functional hybridisation models which are able to handle electron correlation accurately, and also the importance of lattice effects.

 Please wait while we load your content...
Please wait while we load your content...