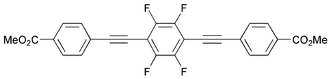
Sonogashira cross-coupling reactions of 4-ethynylmethylbenzoate (1) with aryl halides gave 1,2-bis(4-carbomethoxyphenyl)ethyne (2), 1,4-bis(4-carbomethoxyphenylethynyl)benzene (3), 1,4-bis(4-carbomethoxyphenylethynyl)tetrafluorobenzene (4) and 9,10-bis(4-carbomethoxyphenylethynyl)anthracene (5), protected precursors to conjugated rigid rod bis(carboxylato) ligands. The compound 1,4-bis(4-carbomethoxyphenyl)butadiyne (6) was prepared by the oxidative homo-coupling of 1. The hydrolysis of 2 and 3 by NaOH in methanol gave the sodium salts of the respective carboxylate anions and subsequent treatment with HCl gave the corresponding free carboxylic acids. Compounds 2–6 were characterised by absorption, emission, IR, NMR and mass spectrometry and examined for liquid crystal phase behaviour by differential thermal analysis (DTA) and transmitted polarised light microscopy (TPLM). Compound 4 exhibited a nematic phase stable from 233.6 °C until decomposition at 320 °C. The single-crystal structures of 2, 5 and 6 were determined by X-ray diffraction at 110–160 K.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...