Crystal structure and magnetic properties of a pseudo-cubic close-packed array of oxalate linked {FeII6(μ3-OH)6}6+ clusters
Abstract
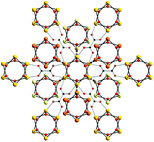
The ionic hexanuclear cluster aggregate [FeII6(μ3-OH)6]6+ has been synthesised using hydrothermal conditions starting from ferrous oxalate in the presence of barium and bromide or iodide ions. A single crystal X-ray structure on the compond Ba4[FeII6(μ3-OH)6(C2O4)6]Br2·6H2O shows that the [FeII6(μ3-OH)6]6+ units are held together by bridging oxalates in a pseudo-cubic close-packed array. The

 Please wait while we load your content...
Please wait while we load your content...