Activity of water in aqueous systems; A frequently neglected property
Abstract
In this critical review, the significance of the term ‘activity’ is examined in the context of the properties of aqueous solutions. The dependence of the activity of water(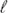 ) at ambient pressure and 298.15 K on solute molality is examined for aqueous solutions containing neutral solutes, mixtures of neutral solutes and salts. Addition of a solute to water(
) at ambient pressure and 298.15 K on solute molality is examined for aqueous solutions containing neutral solutes, mixtures of neutral solutes and salts. Addition of a solute to water(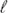 ) always lowers its thermodynamic activity. For some solutes the stabilisation of water(
) always lowers its thermodynamic activity. For some solutes the stabilisation of water(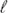 ) is less than and for others more than in the case where the thermodynamic properties of the aqueous solution are ideal. In one approach this pattern is accounted for in terms of hydrate formation. Alternatively the pattern is analysed in terms of the dependence of practical osmotic coefficients on the composition of the aqueous solution and then in terms of solute–solute interactions. For salt solutions the dependence of the activity of water on salt molalities is compared with that predicted by the Debye–Hückel limiting law. The analysis is extended to consideration of the activities of water in binary aqueous mixtures. The dependence on mole fraction composition of the activity of water in binary aqueous mixtures is examined. Different experimental methods for determining the activity of water in aqueous solutions are critically reviewed. The role of water activity is noted in a biochemical context, with reference to the quality, stability and safety of food and finally with regard to health science.
) is less than and for others more than in the case where the thermodynamic properties of the aqueous solution are ideal. In one approach this pattern is accounted for in terms of hydrate formation. Alternatively the pattern is analysed in terms of the dependence of practical osmotic coefficients on the composition of the aqueous solution and then in terms of solute–solute interactions. For salt solutions the dependence of the activity of water on salt molalities is compared with that predicted by the Debye–Hückel limiting law. The analysis is extended to consideration of the activities of water in binary aqueous mixtures. The dependence on mole fraction composition of the activity of water in binary aqueous mixtures is examined. Different experimental methods for determining the activity of water in aqueous solutions are critically reviewed. The role of water activity is noted in a biochemical context, with reference to the quality, stability and safety of food and finally with regard to health science.

 Please wait while we load your content...
Please wait while we load your content...