Photoisomerisation of dibenzobarrelenes—a facile route to polycyclic synthons†
Abstract
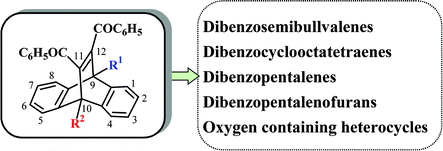
Triplet state mediated di-π-methane rearrangements of dibenzobarrelenes give a variety of interesting synthons, formed as primary and secondary photoproducts. These synthons could find use for the synthesis of complex synthetic targets. This tutorial review highlights the photoisomerisation of some bridgehead substituted dibenzobarrelenes and the products derived from them. Selected examples of photoisomerisations proceeding through a tri-π-methane pathway are also included.

 Please wait while we load your content...
Please wait while we load your content...