Light stability of pyrazolotriazole azamethine dyes at oil/aqueous interfaces
Abstract
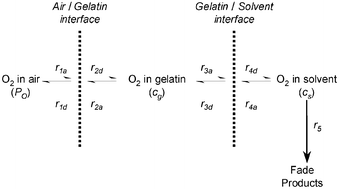
Film and dispersion coatings of two pyrazolotriazole azamethine (PT) dyes are used to study the effect of increasing the surface-area-to-volume (SA ∶ V) ratio of the oil/aqueous (solvent/gelatin) interface on non-oxidative and oxidative fade characteristics. High concentration solutions of the dyes in a poly(vinyl acetate)–dicyclohexylphthalate mixture as solvent are coated on glass substrates with a gelatin overcoat to produce thin film coatings and are also dispersed in gelatin to create “oil-in-gelatin” dispersion coatings. It is calculated that the dispersion coatings possess a SA ∶ V ratio of approximately 20× greater than the film coatings. In non-oxidative fade conditions both the film and dispersion samples possess similar quantum yields of fade, implying that the presence of a solvent/gelatin interface does not significantly affect any electron transfer fade mechanisms. However, in oxidative fade conditions increasing the SA ∶ V ratio of the interface by 20× leads to an increase in the quantum yield of fade of only a factor of 1.5–2. This disproportionate increase in the fade kinetics in dispersion coatings is explained by a kinetic scheme modelling oxygen diffusion through the coatings. It shows that the rate determining step for dye fade starts to become the diffusion of oxygen through the air/gelatin interface, and explains why changes in the SA ∶ V ratio are not fully transferred to the quantum yield of fade.

 Please wait while we load your content...
Please wait while we load your content...