Conformational diastereoisomerism in a chiral pretzelane†
Abstract
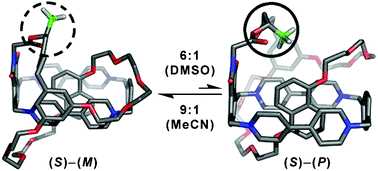
The introduction of a stereogenic center by a stereospecific synthesis into an optically active, donor–acceptor pretzelane, that exhibits helicity as well as fixed chirality, leads to a marked preference for one conformational diastereoisomer over the other in both

 Please wait while we load your content...
Please wait while we load your content...