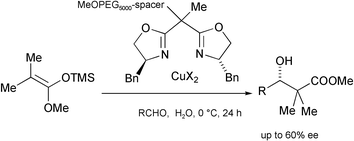
Enantioselective catalysis in water: Mukaiyama-aldol condensation promoted by copper complexes of bisoxazolines supported on poly(ethylene glycol)
Abstract
(S)-3-Phenyl-2-aminopropanol-derived bisoxazolines supported on a modified poly(ethylene glycol) were shown to be effective Cu(II)

 Please wait while we load your content...
Please wait while we load your content...