Compelling evidence for a stepwise mechanism of the alkaline cyclisation of uridine 3′-phosphate esters†‡
Abstract
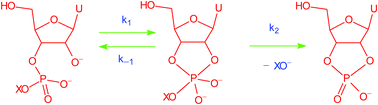
A Brønsted graph with a convex break at pKa (Lg) = 12.58 provides compelling evidence for an intermediate in the alkaline cyclisation of uridine 3′-phosphate esters. The transient pentacoordinated oxyphosphorane dianion intermediate collapses to reactant and cyclic uridine 2′,3′-monophosphate faster than it can pseudo-rotate and isomerise to the 2′-isomer.

 Please wait while we load your content...
Please wait while we load your content...