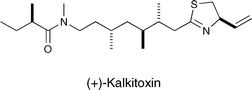
Total synthesis and biological evaluation of (+)-kalkitoxin, a cytotoxic metabolite of the cyanobacterium Lyngbya majuscula†
Abstract
(+)-Kalkitoxin, a metabolite of the marine cyanobacterium Lyngbya majuscula, was synthesized from (R)-2-methylbutyric acid, (R)-cysteine, and (3S, 4S, 6S)-3,4,6-trimethyl-8-(methylamino)octanoic acid. A key step in the synthesis was installation of the anti,anti methyl stereotriad by means of a tandem asymmetric conjugate addition of an organocopper species to an α,β-unsaturated N-acyl oxazolidin-2-one followed in situ by α-methylation of the resultant enolate. The thiazoline portion of kalkitoxin was assembled by titanium tetrachloride catalyzed cyclization of a vinyl substituted amido thiol.

 Please wait while we load your content...
Please wait while we load your content...