Thermodynamic study of sesamol, piperonyl alcohol, piperonylic acid and homopiperonylic acid: a combined experimental and theoretical investigation
Abstract
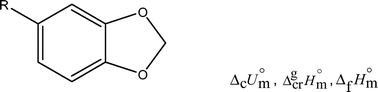
The standard (po
= 0.1 MPa) molar energies of
The most stable geometries of all the compounds were obtained using the density functional theory with the B3LYP functional and two basis sets: 6-31G** and 6-311G**. The nonplanarity of the molecules was analyzed in terms of the anomeric effect, which is believed to arise from the interaction between a nonbonded oxygen p orbital and the empty orbital σ*CO involving the other oxygen atom.
Calculations were performed to obtain estimates of the enthalpies of formation of all the

 Please wait while we load your content...
Please wait while we load your content...