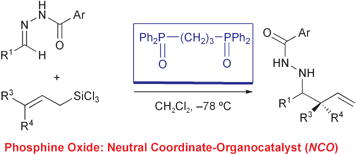
Phosphine oxides were found to be efficient neutral coordinate-organocatalysts (NCOs) for the allylation of N-acylhydrazones. Among the phosphine oxides tested, a three carbon-tethered bisphosphine oxide (dppp dioxide) was found to be the most effective, and in the presence of dppp dioxide, less reactive aromatic and α,β-unsaturated N-acylhydrazones underwent allylation as well as diastereoselective crotylation. Furthermore, a polymer-supported phosphine oxide was also developed as an effective immobilized NCO.