Reactive resin facilitated preparation of an enantiopure fluorobicycloketone
Abstract
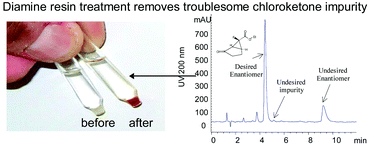
A facile preparation of enantiopure ethyl (1S,5S,6S)-6-fluoro-2-oxobicyclo[3.1.0]hexane-6-carboxylate 1 is described. The key feature of the synthesis involves copper-catalyzed enantioselective intramolecular cyclopropanation of a diazoketone to form endo-fluorocyclopropane 1 in a single operation. Removal of a problematic chloroketone impurity using a reactive resin treatment enabled a high throughput enantiopurity upgrade by chiral HPLC. The development of a scalable synthesis of 1 is presented, including details of the selection of catalyst and ligand optimization, incorporation of a reactive resin treatment and selection of chiral HPLC media and conditions.

 Please wait while we load your content...
Please wait while we load your content...