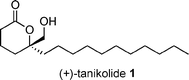
Total synthesis of (+)-tanikolide via oxidative lactonization
Abstract
(+)-Tanikolide has been synthesized in eight linear steps with a 31% overall yield. The key step in the synthesis utilizes a recently developed tandem oxidative cleavage–lactonization of a precursor alkenol to deliver the lactone moiety.

 Please wait while we load your content...
Please wait while we load your content...