Structure–activity relationships of pyrithiones – IPC-81 toxicity tests with the antifouling biocide zinc pyrithione and structural analogs
Abstract
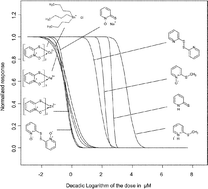
Zinc pyrithione (1-hydroxypyridine-2-thione, zinc complex; ZnPT2) is currently viewed as the top prospect for replacing tributyltin antifoulants in ship paints. Thus, the risk assessment of a high scale release of ZnPT2 to the natural environment is of increasing importance. The knowledge of the molecular mechanisms related to biological effects of ZnPT2 and its transformation products is crucial for this assessment and thus for the decision whether pyrithiones are sound or “green” alternatives to organotin antifoulants. A multitude of biological effects of pyrithiones is already known while the underlying molecular mechanisms of action remain obscure. This study presents toxicological data of zinc pyrithione and several structural analogs in rat leukemic cells (IPC-81). The N-hydroxythioamide functional group proved to play a significant role in the molecular mechanisms related to the biological action. Structural analogs, which are deprived of one or more molecular interaction or chemical reaction potentials given by this group (namely pyridine, pyridine 1-oxide and pyridine 2-thione, bis(2-pyridinyl)disulfide, and three methylated metabolites), exhibit far less toxic potential in IPC-81 cells than pyrithiones (i.e., 2-pyridinethione-1-oxides). In particular the trans-metallization products of ZnPT2, iron (FePT3) and copper (CuPT2) pyrithione, and the oxidation product bis(2-pyridinyl)disulfide 1,1′-dioxide (pyrithione disulfide, (PT2)) have been proven to be as toxic as ZnPT2 and tributyltin chloride in IPC-81 cells. CuPT2, FePT3 and (PT)2 need to be considered as environmental transformations products of ZnPT2.

 Please wait while we load your content...
Please wait while we load your content...