Structure–reactivity correlations in the selective aerobic oxidation of cinnamyl alcohol: in situ XAFS
Abstract
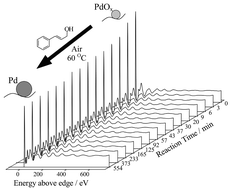
The structural evolution of a Pd/C catalyst during the liquid phase selective aerobic oxidation of cinnamyl alcohol has been followed by in situ XAFS and XPS. The fresh catalyst comprised highly dispersed, heavily oxidised Pd particles. Cinnamyl alcohol oxidation resulted in the rapid reduction of surface palladium oxide and a small degree of concomitant particle growth. These structural changes coincided with a large drop in catalytic activity. Prereduced Pd/C exhibited a significantly lower initial oxidation rate demonstrating the importance of surface metal oxide in effecting catalytic oxidation. Use of a Pd black model system confirmed that the oxide→metal transformation was the cause, and not result, of catalyst deactivation.

 Please wait while we load your content...
Please wait while we load your content...