Water-soluble, relatively light-stable, chiral and achiral silver(I) complexes {[Ag2(ca)2]}n and {[Ag2(ca)2(Hca)2]}n
(R- and S-Hca =
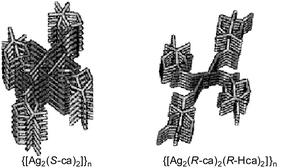
(1R,4S)- and (1S,4R)-4,7,7-trimethyl-3-oxo-2-oxabicyclo[2.2.1]heptane-1-carboxylic acid, respectively) prepared from the reaction of Ag2O with chiral and racemic Hca in 1 ∶ 2 and 1 ∶ 4 molar ratios were characterized by elemental analysis, TG/DTA, FTIR, and solution (1H, 13C and 109Ag) and solid-state (13C) NMR spectroscopy. Crystallography revealed that unique 21 helical polymer and zigzag structures were formed on self-assembly of the dimeric units in the crystals of {[Ag2(S-ca)2]}n and three {[Ag2(ca)2(Hca)2]}n. In the crystal of {[Ag2(S-ca)2]}n two 21 helices and a loop were observed in the stair-like polymer structure, whereas zigzag and a loop were seen in the crystals of three {[Ag2(ca)2(Hca)2]}n. Carbon NMR spectra in the solid state and in D2O indicated that these polymeric structures were loosely bound and fast ligand-exchange reactions took place in aqueous solution. The complexes, {[Ag2(ca)2]}n and {[Ag2(ca)2(Hca)2]}n, showed a wide spectrum of effective antimicrobial activity as anticipated for weak silver(I)–O bonding complexes. Similar antimicrobial activity of {[Ag2(ca)2]}n and {[Ag2(ca)2(Hca)2]}n against selected microorganisms suggested that ligand exchangeability played an important role as well as the coordination geometry of the silver(I) ion.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...