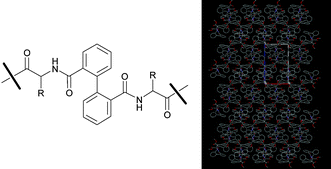
Crystallization-induced dynamic resolution and analysis of the non-covalent interactions in the crystal packing of peptide–biphenyl hybrids†‡
Abstract
Dynamic atropselective resolutions of the
- This article is part of the themed collection: New Trends in Crystal Engineering

 Please wait while we load your content...
Please wait while we load your content...