Synthesis and resolution of the planar chirality of ester-functionalised phospharuthenocenes
Abstract
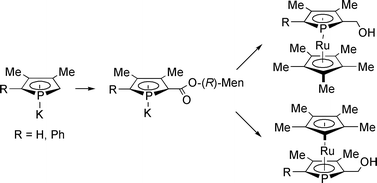
Menthyl phospholide ester anions provide an operationally simple and high yielding entry to the first planar chiral enantiopure phospharuthenocene derivatives.

 Please wait while we load your content...
Please wait while we load your content...