Diastereospecific synthesis of phosphinidene-capped cyclodextrins leading to “introverted” ligands†
Abstract
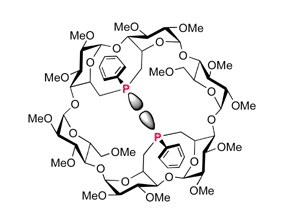
α-Cyclodextrins (α-CDs) containing “PPh” units which cap the primary face of the CD were obtained in high yield by reaction of Li2PPh with A,B- or A,C-dimesylated and A,B,D,E-tetramesylated precursors; the resulting

 Please wait while we load your content...
Please wait while we load your content...