Photoreactions of p-benzo-, p-naphtho- and p-anthraquinones with ascorbic acid
Abstract
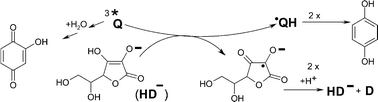
The photoreduction of 1,4-benzoquinone (BQ), 1,4-naphthoquinone (NQ), 9,10-anthraquinone (AQ) and several derivatives, e.g. dimethylBQ, trimethylBQ, duroquinone, bromoNQ, methoxyNQ, methylAQ and dimethylAQ in acetonitrile–water by ascorbate was studied by time-resolved UV-vis spectroscopy using 20 ns laser pulses at 308 nm and continuous 254 nm irradiation. The semiquinone radical (˙QH/Q˙−) is formed after H-atom transfer from ascorbate to the quinone triplet state. The rate constant for quenching is kq = (2–9) × 109 M−1 s−1. Termination of the radicals takes place in the µs–ms range. The results are compared with those initiated by electron transfer from DABCO under similar conditions, where the kq values are similar, but the termination of Q˙− takes place by electron back transfer not yielding hydroquinones. Specific properties of the quinone triplet state, e.g. self-quenching, nucleophilic water addition and the effects of structure are discussed.

 Please wait while we load your content...
Please wait while we load your content...