New nucleoside analogs from 2-amino-9-(β-d-ribofuranosyl)purine
Abstract
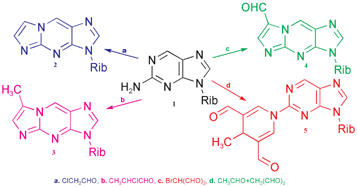
Four novel derivatives of 2-amino-9-(β-D-ribofuranosyl)purine (1) were synthesised and fully characterised. When 1 was reacted with chloroacetaldehyde (a), 2-chloropropanal (b), bromomalonaldehyde (c) and a mixture of chloroacetaldehyde + malonaldehyde (d), 3-(β-D-ribofuranosyl)-imidazo-[1,2a]purine (2), 3-(β-D-ribofuranosyl)-5-methylimidazo-[1,2a]purine (3), 3-(β-D-ribofuranosyl)-5-formylimidazo-[1,2a]purine (4) and 9-(β-D-ribofuranosyl)-2-(3,5-diformyl-4-methyl-1,4-dihydro-1-pyridyl)purine (5) were formed, respectively. The products were isolated, purified by chromatography and characterised by MS, complete NMR assignment as well as fluorescence and UV spectroscopy. The yields of these reactions were moderate (14–20%). The fluorescence properties differed from those of the starting compound and the quantum yields were considerably lower.

 Please wait while we load your content...
Please wait while we load your content...