Alkaline-earth substituted lanthanum manganites La1 −
xAxMnO3
(A = Ca, Sr; x
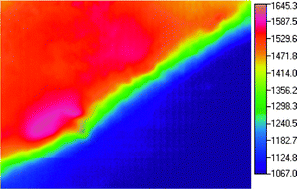
= 0, 0.4) were synthesized in air by self-propagating high-temperature synthesis (SHS): a combustion process involving the reaction of lanthanum(III) oxide, calcium oxide (or carbonate), strontium peroxide, manganese metal powder and sodium perchlorate. A very sensitive thermal imaging method was used to study the combustion characteristics of the SHS-process. This method is based on the continuous measurement of the combustion process using an IR-camera and software developed by MIKRON Instrument Co., Inc. (M9100 Pyrovision Series – Imaging Pyrometer). X-Ray powder diffraction, scanning electron microscopy (SEM)/energy dispersive analysis by X-rays (EDAX), infrared spectroscopy and vibrating sample magnetometry were carried out on all samples. X-Ray diffraction data showed that single-phase orthorhombic LaMnO3 and monoclinic La0.6Ca0.4MnO3 and La0.6Sr0.4MnO3 were formed. Magnetic properties were found to transform from antiferromagnetic for LaMnO3 to weak ferromagnetic for La0.6Ca0.4MnO3 to strong ferromagnetic for La0.6Sr0.4MnO3. The experimental results show the SHS route is useful for the synthesis of multicomponent Mn-based oxides. The thermal imaging work enables accurate monitoring of the SHS combustion process and enabled velocity of propagation, maximum temperature, cooling rates, synthesis wave width and synthesis wave path to be determined with high precision.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...