Solid-to-solid biocatalysis: thermodynamic feasibility and energy efficiency
Abstract
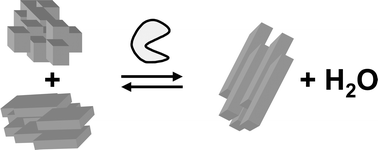
Enzymes can catalyse solid-to-solid condensation reactions in highly concentrated aqueous substrate suspensions. Reaction products precipitate from the reaction mixture and very high conversion yields can be obtained in low volume reactors. Solid-to-solid biocatalysis combines the advantages of using enzymes in aqueous media with the high conversion yields that are typically associated with non-aqueous biocatalysis. In this article, methods are presented for the calculation of the Gibbs free energy changes and heats of reaction of condensation reactions to form amides. The overall enthalpy change of the enzymatic reaction was compared to that of the conventional chemical methods and it was found that the enzymatic reaction produces a third of the heat with better atom efficiency.

 Please wait while we load your content...
Please wait while we load your content...