Alkyl–η2-alkene niobocene and tantalocene complexes with the allyldimethylsilyl–η5-cyclopentadienyl ligand: synthesis, NMR studies and DFT calculations†
Abstract
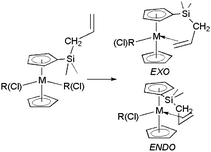
Group 5 metal complexes [M(η5-C5H5){η5-C5H4SiMe2(CH2-η2-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)}X]
(M = Nb, X = Me, CH2Ph, CH2SiMe3; M = Ta, X = Me, CH2Ph) and [Ta(η5-C5Me5){η5-C5H4SiMe2(CH2-η2-CH
CH2)}X]
(M = Nb, X = Me, CH2Ph, CH2SiMe3; M = Ta, X = Me, CH2Ph) and [Ta(η5-C5Me5){η5-C5H4SiMe2(CH2-η2-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)}X]
(X = Cl, Me, CH2Ph, CH2SiMe3) containing a chelating
CH2)}X]
(X = Cl, Me, CH2Ph, CH2SiMe3) containing a chelating ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)}Cl2]
(Cp = C5H5, M = Nb, Ta, Cp = C5Me5, M = Ta). These chloro– and
CH2)}Cl2]
(Cp = C5H5, M = Nb, Ta, Cp = C5Me5, M = Ta). These chloro– and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)}XL]
(X = Cl, Me, CH2Ph, CH2SiMe3). Reaction of the
CH2)}XL]
(X = Cl, Me, CH2Ph, CH2SiMe3). Reaction of the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)}H(NtBu)].
CH2)}H(NtBu)].

 Please wait while we load your content...
Please wait while we load your content...