Unexpected formation of a copper(ii) 12-metallacrown-4 with (S)-glutamic-γ-hydroxamic acid: a thermodynamic and spectroscopic study in aqueous solution
Abstract
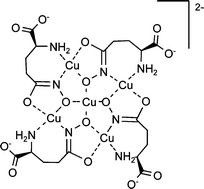
The equilibria of copper(II) with (S)-glutamic-γ-hydroxamic acid (H2L) were investigated in aqueous solution by different techniques: glass

 Please wait while we load your content...
Please wait while we load your content...