Photochromism and thermochromism of Schiff bases in the solid state: structural aspects
Abstract
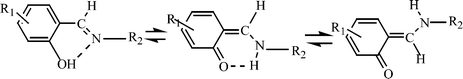
This tutorial review describes in a brief historical perspective the most important organic compounds that exhibit photochromism in the crystalline state since its discovery in 1867 up to now and considers in detail

 Please wait while we load your content...
Please wait while we load your content...