What is the nature of the long bond in the TCNE22− π-dimer?
Abstract
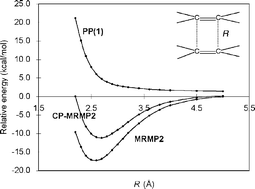
The TCNE22− π-dimer has many of the characteristics of a chemical bond despite its remarkably long (2.9 Å) C–C bondlength, as it exists in crystalline solids. Using computational methods, we examine the nature of this long bond by obtaining potential energy curves that are, for the first time, in satisfactory agreement with recent experiments. We find that the unusually long C–C bond observed in π-TCNE22− dimers is in fact an outcome of significant dispersion attractions between the two cofacial monomers, as well as their (partial) intradimerπ-bonding interactions.

 Please wait while we load your content...
Please wait while we load your content...