1,2-Chlorine atom migration in 3-chloro-2-butyl radicals: a computational study†
Abstract
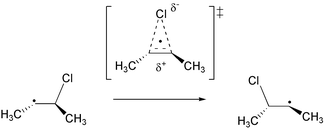
Skell’s hypothesis of bridged 2-haloalkyl radicals has been revisited using several different theoretical methods and the 3-chloro-2-butyl radical as an example.
The structure as well as several unimolecular reaction pathways of the 3-chloro-2-butyl radical 1 have been studied at several different theoretical levels (B3LYP/aug-cc-pVDZ, BHLYP/aug-cc-pVDZ, G3(ROMP2)B3). The symmetrically chlorine-bridged structure is a transition state at all levels of theory and the most favorable ground state structure is the unbridged β-chloroalkyl radical. Reaction barriers for the 1,2-chlorine migration process are higher than those for rotation around the central C–C bond. 1,2-migration of the chlorine atom is accompanied by an increase in chlorine negative charge as well as chlorine spin density. This hybrid homo-/heterolytic process is well known from rearrangement reactions in β-(dialkoxyphosphoryloxy)alkyl radicals and suggests that chlorine migration can be influenced by polar substituent effects.

 Please wait while we load your content...
Please wait while we load your content...