Concise synthesis of (±)-horsfiline and (±)-coerulescine by tandem cyclisation of iodoaryl alkenyl azides†
Abstract
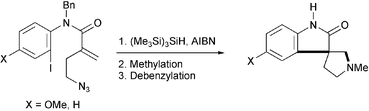
A brief, efficient and economical synthesis of the spiropyrrolidinyloxindoles, horsfiline and coerulescine, has been achieved, starting from

 Please wait while we load your content...
Please wait while we load your content...