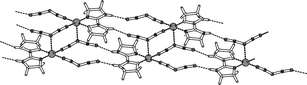
The synthesis and crystal structures, as well as the electronic and magnetic properties, of four new compounds containing the ligands 2,2′-biimidazoline (biz) and dicyanamide (dca) with the general formula M(II)(biz)x(dca)2
(where M = Cu(II), Co(II), Ni(II) and x = 1,2) are reported. In the compound Cu(biz)(dca)2(1), the equatorial plane around the Cu(II) atom is formed by two nitrogen atoms of one biz ligand and two nitrile atoms of two dca molecules. The dca anions are connected to a neighbouring Cu atom, with the central amide nitrogen acting as the axial atom, forming in this way a 2D polymeric sheet. The compounds Cu(biz)2(dca)2
(2) and Co(biz)2(dca)2
(3), which are isostructural, contain octahedral metal ions with the basal plane occupied by four nitrogen atoms of two biz ligands and the axial positions formed by two amide nitrogen atoms of monodentate dca ligands. The compound Ni(biz)2(dca)2
(4), differs from 2 and 3, as in this case the axial positions are occupied by the nitrile nitrogen atoms of a monodentate dca ligand. The main difference between the Co and the Cu compounds is the axial M–N bond, which in the case of Cu is elongated to 2.60 Å, due to the Jahn–Teller effect. The coordination of the metal atom via the amide nitrogen atoms of dca has so far rarely been observed. All four compounds also show quite different hydrogen bonding systems via the N–H groups of the biz ligands and the nitrile nitrogen atoms of the dca molecules, forming interesting 3D and 2D polymeric and sheet-like arrays. The infrared absorptions of the compounds, as well as the electronic and EPR absorptions, are in good agreement with the crystal structures obtained. Magnetic susceptibility measurements revealed that no significant (J > −1 cm−1) interactions are present between the metal atoms, as was expected, since no serious overlap of the magnetic metal orbitals takes place via the dca ligands.

 Please wait while we load your content...
Please wait while we load your content...