Selective palladium-catalysed dimerisation of methyl acrylate in ionic liquids: towards a continuous process†
Abstract
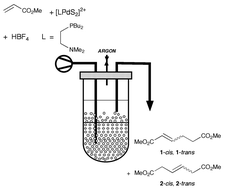
The activity and stability of cationic palladium complexes [Pd(PBu3)2S2]2+ used for the selective tail-to-tail dimerisation of methyl acrylate are significantly improved with the utilisation of ionic liquids like [BMIM][BF4] or the protonated N-butyl-imidazole, [HBIM][BF4]. Problems related to product inhibition and catalyst recycling are overcome by running the reaction in a two-phase mode, toluene being used as extractant. Catalyst stabilisation is further improved by trapping the ancillary ligand into the ionic liquid with an ionic tail: with the use of 1-dibutylphosphino-2-dimethylaminoethane, the catalyst is stable for more than 100 h, therefore demonstrating the feasibility of a continuous process for methyl acrylate dimerisation.

 Please wait while we load your content...
Please wait while we load your content...