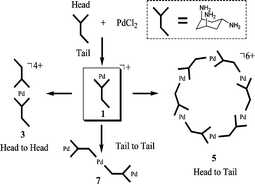
The reaction of cis,trans-1,3,5-triaminocyclohexane·3HX (L·3HX) with PdX2
(X = Br, Cl) affords a wide range of coordination complexes that represent the different coordination modes available to L. Monoligand complexes [Pd(LH)Cl2]Cl (1) and [Pd(LH)Br2]2[PdBr4]
(2) demonstrate the bidentate coordination of L with the two cis amino ‘head’ groups chelating the palladium(II) ion and the third trans amino ‘tail’ group being protonated. Diligand complexes [Pd(LH)2]X (X =
(NO3)43, (SO4)24) show a ‘head-to-head’ coordination mode with the protonated trans amino groups adopting a conformation that positions them opposite to each other. Both sets of amino groups are engaged in coordination in a cyclic ‘head-to-tail’ fashion found in the hexanuclear ring clusters [{Pd(L)X}6]X6
(X = Cl 5, Br 6). 5 and 6 are isostructural, both in the solid state and in solution, despite accommodating six chloro or bromo ligands into the cluster framework. A trinuclear complex [Pd{Pd(L)Cl2}2Cl2]
(7) reveals ‘tail-to-tail’ coordination of two ligands for the centre palladium(II) ion in addition to their ‘head’ amino groups individually chelating other palladium(II) ions. Complexes 1–7 were characterised by single-crystal X-ray diffraction, elemental analysis, IR and by NMR spectroscopy (1–6).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...