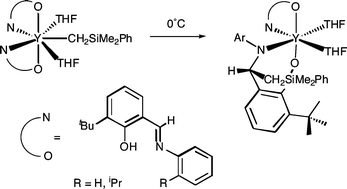
Scandium and yttrium bis(ligand) mono(alkyl) complexes, of N-phenyl (L1) N-2-isopropylphenyl (L2) and N-mesityl (L3) substituted ortho-tert-butylsalicylaldiminato ligands were prepared by alkane elimination from [M(CH2SiMe2R)3(THF)2]
(R = Me, Ph) and two equivalents of proteo ligand (HL). The resulting [L2M(THF)n(CH2SiMe2R)]
(n
= 0–2, L = L1
(1), L2
(2), L3
(3)) complexes are thermally unstable, decomposing rapidly between −20 and 20 °C. In order to gain insight in to ligand features necessary to impart thermal stability in early transition metal organometallic chemistry, the decomposition pathways of 1–3 have been investigated and compared with more sterically congested N-2,6-diisopropylphenyl substituted analogues. Compounds 1 and 2 decompose rapidly and cleanly at room temperature by 1,3-migration of the entire CH2SiMe2R group to the aldimine carbon. By contrast, L3 mono(alkyls)
3 decompose cleanly by metallation of an ortho-C6H2Me3 group. In the case of yttrium, the metallated alkyl undergoes subsequent 1,3-migration to the aldimine carbon, forming a five-membered C4N-ring.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...