Bridge-splitting kinetics, equilibria and structures of trans-biscyclooctene complexes of platinum(ii)†
Abstract
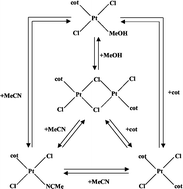
Cyclooctene (C8H14, cot) complexes of platinum(II) have been synthesised and characterised by means of multinuclear NMR, UV-Vis spectroscopy and X-ray crystallography. The bridge-splitting equilibrium constants of the dinuclear, chloride-bridged trans-[PtCl2(cot)]2, 1, in dichloromethane solvent at 298 K with MeOH, MeCN and cot, yielding trans-[PtCl2(cot)(MeOH)], 2, trans-[PtCl2(cot)(MeCN)], 3, and trans-[PtCl2(cot)2], 4, were determined by UV-Vis measurements as K12
= 0.0169 ± 0.0015, K13
= 9.7 ± 0.9 and K14
= 2.05 ± 0.06 mol−1 dm3, respectively. Substitution of one cyclooctene from 4 by MeOH gives K42
= 0.110 ± 0.009 and by MeCN K43
= 2.25 ± 0.03. The bis-cyclooctene complexes 1 and 4 react quantitatively with chloride to give [PtCl3(cot)]−, 5. The kinetics for bridge-splitting of 1 with MeOH, MeCN and cot was studied by conventional and cryo-stopped-flow spectroscopy. Second-order rate constants at 298 K are 0.128 ± 0.003, 4.93 ± 0.02 and 0.0637 ± 0.0009 mol−1 dm3 s−1, respectively. The corresponding activation parameters are ΔH≠
= 43.7 ± 1.8, 42.0 ± 0.8 and 39.6 ± 0.9 kJ mol−1 and ΔS≠
=
−115 ± 6, −91 ± 3 and −135 ± 3 J K−1 mol−1, indicating associative activation with a relatively large -TΔS≠ contribution to ΔG≠. Crystal and molecular structures of 1, 4, and the tetraphenylphosphonium salt of 5 have been determined, indicating a moderate ground-state trans influence of cyclooctene. The molecular structure of 4 with two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds coordinated trans to each other and perpendicular to the platinum coordination plane, features a significant Pt–C bond lengthening compared to cyclooctene complexes with chloride or nitrogen trans to cyclooctene, leading to high reactivity and thermodynamic instability. Equilibrium data confirm that the thermodynamic stability of a cyclooctene coordinated trans to another cyclooctene is much lower than trans to a ligand with less π-back-bonding capacity.
C double bonds coordinated trans to each other and perpendicular to the platinum coordination plane, features a significant Pt–C bond lengthening compared to cyclooctene complexes with chloride or nitrogen trans to cyclooctene, leading to high reactivity and thermodynamic instability. Equilibrium data confirm that the thermodynamic stability of a cyclooctene coordinated trans to another cyclooctene is much lower than trans to a ligand with less π-back-bonding capacity.

 Please wait while we load your content...
Please wait while we load your content...