Equilibria and dynamics of some aqueous peroxomolybdophosphate catalysts: a potentiometric and 31P NMR spectroscopic study
Abstract
The equilibrium speciation in the p H+
+
q
H+
+
q MoO42−
+
r
MoO42−
+
r H2O2
+
s
H2O2
+
s H2PO4−
+
t
H2PO4−
+
t SO42−
⇌
(H+)p(MoO42−)q(H2O2)r(H2PO4−)s(SO42−)tp
SO42−
⇌
(H+)p(MoO42−)q(H2O2)r(H2PO4−)s(SO42−)tp −
− 2q
2q −
− s
s −
− 2t system in 0.300 M Na2(SO4) medium at 25 °C and [H2O2]tot/[Mo]tot > 2 has been determined from potentiometric data and
2t system in 0.300 M Na2(SO4) medium at 25 °C and [H2O2]tot/[Mo]tot > 2 has been determined from potentiometric data and  −
− 2q
2q −
− s
s −
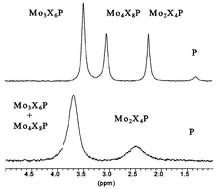
− 2t. The numbers in parentheses refer to the values of p, q, r, s and t in the formula above. The following formation constants with 3σ were obtained; log β0,1,2,1,0
= 5.16 ± 0.09, log β1,1,2,1,0
= 12.73 ± 0.02 (pKa
= 7.63), log β2,1,2,1,0
= 16.14 ± 0.03 (pKa
= 3.42), log β2,2,4,1,0
= 25.03 (± 0.04), log β3,2,4,1,0
= 29.54 ± 0.02 (pKa
= 4.51), log β4,3,6,1,0
= 42.30 ± 0.03, log β5,3,6,1,0
= 44.06 ± 0.08 (pKa
= 1.76), log β6,4,8,1,0
= 57.30 ± 0.07. pKa values for H3PO4 and H2PO4− were determined to 2.00 and 6.48, respectively. Chemical exchange processes were detected by
2t. The numbers in parentheses refer to the values of p, q, r, s and t in the formula above. The following formation constants with 3σ were obtained; log β0,1,2,1,0
= 5.16 ± 0.09, log β1,1,2,1,0
= 12.73 ± 0.02 (pKa
= 7.63), log β2,1,2,1,0
= 16.14 ± 0.03 (pKa
= 3.42), log β2,2,4,1,0
= 25.03 (± 0.04), log β3,2,4,1,0
= 29.54 ± 0.02 (pKa
= 4.51), log β4,3,6,1,0
= 42.30 ± 0.03, log β5,3,6,1,0
= 44.06 ± 0.08 (pKa
= 1.76), log β6,4,8,1,0
= 57.30 ± 0.07. pKa values for H3PO4 and H2PO4− were determined to 2.00 and 6.48, respectively. Chemical exchange processes were detected by

 Please wait while we load your content...
Please wait while we load your content...