Synthesis, structure, and preliminary magnetic studies of unprecedented hexacopper(ii) barrel clusters with spin ground state S = 3
Abstract
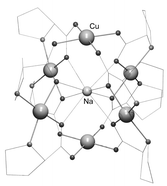
The structure of a discrete hexacopper(II) barrel cluster and that of a coordination polymer formed by a related hexanuclear repeating unit have been determined. The CuII metals of the hexanuclear units are held together by eight chelating L-prolinato type ligands, which in turn provide the eight oxygen donors trapping a sodium ion in the center. The structure of the [Cu6Na] unit in the discrete system and in the infinite cluster-chain are essentially the same and both display intra-unit ferromagnetic superexchange. A preliminary magnetic study shows that, for both compounds, the copper centers within the [Cu6] unit are ferromagnetically coupled, yielding an S = 3 spin ground state.

 Please wait while we load your content...
Please wait while we load your content...