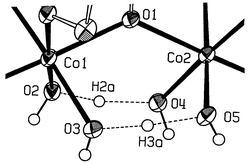
The dicobalt(III) complex, [Co2(bpbp)(μ-H3O2)2](ClO4)3
(bpbp−
= 2,6-bis[bis(2-pyridylmethyl)aminomethyl]-4-tert-butylphenolate), obtained by reaction of cobalt(II) perchlorate with Hbpbp under ambient conditions contains two μ-H3O2− bridging ligands. The H-bonded O⋯O distances in this motif are 2.437(3) and 2.456(4)
Å, respectively, with a Co⋯Co separation of 3.601(1)
Å. The structurally precedented peroxo bridged complexes, [Co2(bpbp)(μ-O2)(μ-RCO2)](ClO4)2
(R = CH3 or C6H5), are formed if a carboxylate is present. The X-ray crystal structure showed O–O and Co⋯Co distances of 1.422(3) and 3.168(1)
Å, respectively, in the case of R = CH3. ESI-MS shows that the bridging peroxo ligand is easily eliminated from [Co2(bpbp)(μ-O2)(μ-CH3CO2)]2+, m/z 390.0, as implicated by the observation of [Co2(bpbp)(μ-CH3CO2)]2+, m/z 374.1, corresponding to loss of the mass equivalent of dioxygen. The superoxo complex [Co2(bpbp)(μ-O2)(μ-CH3CO2)]3+ can be prepared by Ce(IV) oxidation of [Co2(bpbp)(μ-O2)(μ-CH3CO2)]2+. Reaction of [Co2(bpbp)(μ-H3O2)2]3+ with hydrazine in air gives the dicobalt(II) complex [Co2(bpbp)(NH2NHCO2)2]ClO4. In this complex the two exogenous hydrazinecarboxylato ligands are bound to individual metal ions with weak H-bonding between them as shown by X-ray structure analysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...