The iron phosphates [C4H12N2][FeII(H2O)6](HPO4)2
(1), 3∞{[NH4][FeIII2(OH)(PO4)2(H2O)]·H2O}
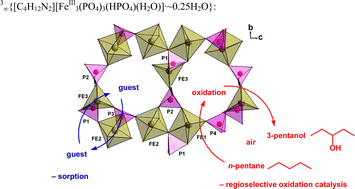
(2) and 3∞{[C4H12N2][FeIII3(PO4)3(HPO4)(H2O)]·∼0.25H2O}, (3) were synthesized by hydrothermal methods and their single-crystal X-ray structures were determined. While compound 1 is only an extended hydrogen bonded network of its three ionic building blocks, compounds 2 and 3 are three-dimensional open-framework materials albeit of different porosity. The structure of 2 corresponds to the mineral sphenicidite. The iron building blocks in 3 are pairs of distorted edge-sharing {FeO6} octahedra and a five-coordinated iron atom, {FeO5}, with a mostly trigonal-bipyramidal polyhedron. The oxidation-state assignment of 2 was backed by 57Fe Mössbauer spectroscopy. Thermogravimetric analysis (TGA) of 2 and 3 shows clearly separated steps of weight loss due to the loss of water of crystallization, aqua ligand and amine template molecules. X-ray powder diffractometry proved that the empty host-frameworks were still intact after heating to 215 °C. The porous empty frameworks of 2 and 3 could be employed as sorbents towards alkanes, alcohols, chlorinated halocarbons, amines and ethers. The larger-porous framework of 3
(but not that of 2) was found to be a catalyst for the highly regioselective oxidation of n-pentane to 3-pentanol with air at 15 bar and 100 °C.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...