Supramolecular stereoisomer – the conformational isomer of 1,1,6,6-tetraphenylhexa-2,4-diyne-1,6-diol in the different inclusion compounds
Abstract
The host  +
+ (2) and (1)
(2) and (1) +
+ (3) are 1∶2 and 2∶1, respectively. In the complex of (1)
(3) are 1∶2 and 2∶1, respectively. In the complex of (1) +
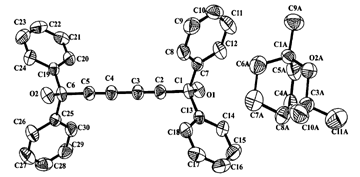
+ (2), the host (1) is linked with (2) via hydrogen bonding and exhibits antiperiplanar conformation, whereas in the 2∶1 host–guest complex (1)
(2), the host (1) is linked with (2) via hydrogen bonding and exhibits antiperiplanar conformation, whereas in the 2∶1 host–guest complex (1) +
+ (3), hosts (1) are associated with each other by hydrogen bonding and the complex adopts a gauche conformation; guests contact with hosts by C–H⋯π weak hydrogen bonding. The structures of the two complexes and conformational isomers of the host have been determined by
(3), hosts (1) are associated with each other by hydrogen bonding and the complex adopts a gauche conformation; guests contact with hosts by C–H⋯π weak hydrogen bonding. The structures of the two complexes and conformational isomers of the host have been determined by

 Please wait while we load your content...
Please wait while we load your content...