Synthesis and reactivity of a stable crystalline diastereomerically pure trifluoromethanesulfinic acid derivative: (S)-(−)-1-trifluoromethylsulfinyl-(R)-4-phenyloxazolidin-2-one
Abstract
Efficient synthesis of the title compound, the first diastereomerically pure trifluoromethanesulfinic acid derivative (8), has been achieved by direct trifluoromethanesulfinylation of the lithiated (4R)-(−)-4-phenyloxazolidin-2-one; in contrast to the reaction between CF3S(O)Cl and (1R,2S,5R)-(−)-menthol which occurs with low stereoselectivity (<10% de), 8 affords the O-menthyl trifluoromethanesulfinate derivative in >98% de.
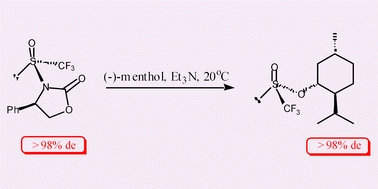

 Please wait while we load your content...
Please wait while we load your content...