The role of the counteranion in the cation-π interaction
Abstract
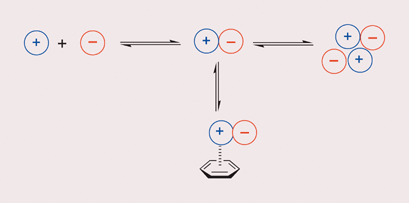
Chemical double mutant cycles have been used to quantify cation-π interactions in chloroform as a function of the nature of the counteranion. The cation-π interaction is −2.5 ± 0.4 kJ mol−1 and independent of the anion, even though the overall stability of the complexes varies by an order of magnitude due to competition of the anion for alternative binding sites.

 Please wait while we load your content...
Please wait while we load your content...