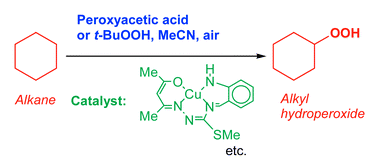
Various copper(I) and copper(II) derivatives, both “simple” ones (copper acetate, perchlorate and a complex with CH3CN) and compounds containing N,O-chelating ligands, catalyse very efficient (turnover numbers attain 2200) oxidation of saturated hydrocarbons with peroxyacetic acid (PAA) or tert-butyl hydroperoxide (TBHP) in acetonitrile solution at 60 °C. Alkyl hydroperoxide, alcohol and ketone are formed, the main product being an alkyl hydroperoxide in the oxidation with PAA and an alcohol for the case of TBHP. It has been proposed that the oxidation with PAA is induced via the attack of species r˙
[HO˙ or CH3C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O˙] on the alkane, RH. A competitive attack of r˙ on the solvent, CH3CN, also occurs. It has been assumed that in the case of the reaction catalysed by complex Cu(CH3CN)4BF4, copper is present mainly in the form of Cu+ cation, and the rate-limiting step of the oxidation process is the formation of r˙
viareaction (1): CH3C(
O)O˙] on the alkane, RH. A competitive attack of r˙ on the solvent, CH3CN, also occurs. It has been assumed that in the case of the reaction catalysed by complex Cu(CH3CN)4BF4, copper is present mainly in the form of Cu+ cation, and the rate-limiting step of the oxidation process is the formation of r˙
viareaction (1): CH3C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OOH + Cu+
→ CH3C(
O)OOH + Cu+
→ CH3C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O˙
+ HO−
+ Cu2+ or/and CH3C(
O)O˙
+ HO−
+ Cu2+ or/and CH3C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OOH + Cu+
→ CH3C(
O)OOH + Cu+
→ CH3C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O−
+ HO˙
+ Cu2+ with initial rate W1
=
k1[PAA][Cu(CH3CN)4BF4] and k1
= 1.7 mol−1 dm3 s−1 at 60 °C. The activity of the Cu-catalyst is dramatically changed on a small modification of N,O-chelating ligands in the catalyst.
O)O−
+ HO˙
+ Cu2+ with initial rate W1
=
k1[PAA][Cu(CH3CN)4BF4] and k1
= 1.7 mol−1 dm3 s−1 at 60 °C. The activity of the Cu-catalyst is dramatically changed on a small modification of N,O-chelating ligands in the catalyst.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O˙] on the
O)O˙] on the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OOH + Cu+
→ CH3C(
O)OOH + Cu+
→ CH3C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O˙
+ HO−
+ Cu2+ or/and CH3C(
O)O˙
+ HO−
+ Cu2+ or/and CH3C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OOH + Cu+
→ CH3C(
O)OOH + Cu+
→ CH3C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O−
+ HO˙
+ Cu2+ with initial rate W1
=
k1[PAA][Cu(CH3CN)4BF4] and k1
= 1.7 mol−1 dm3 s−1 at 60 °C. The activity of the Cu-catalyst is dramatically changed on a small modification of N,O-chelating
O)O−
+ HO˙
+ Cu2+ with initial rate W1
=
k1[PAA][Cu(CH3CN)4BF4] and k1
= 1.7 mol−1 dm3 s−1 at 60 °C. The activity of the Cu-catalyst is dramatically changed on a small modification of N,O-chelating 

 Please wait while we load your content...
Please wait while we load your content...