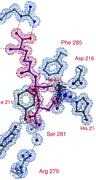
Isopenicillin N synthase (IPNS) catalyses conversion of the linear tripeptide δ-(L-α-aminoadipoyl)-L-cysteinyl-D-valine (ACV) to isopenicillin N (IPN), the central step in biosynthesis of the β-lactam antibiotics. The unsaturated substrate analogue δ-(L-α-aminoadipoyl)-L-cysteinyl-D-vinylglycine (ACvG) has previously been incubated with IPNS and a single product was isolated, a 2-α-hydroxymethyl isopenicillin N (HMPen), formed via a monooxygenase mode of reactivity. ACvG has now been crystallised with IPNS and the structure of the anaerobic IPNS:Fe(II):ACvG complex determined to 1.15 Å resolution. Furthermore, by exposing the anaerobically grown crystals to high-pressure oxygen gas, a structure corresponding to the bicyclic product HMPen has been obtained at 1.60 Å resolution. In light of these and other IPNS structures, and recent developments with related dioxygenases, the [2 + 2] cycloaddition mechanism for HMPen formation from ACvG has been revised, and a stepwise radical mechanism is proposed. This revised mechanism remains consistent with the observed stereospecificity of the transformation, but fits better with apparent constraints on the coordination geometry around the active site iron atom.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...