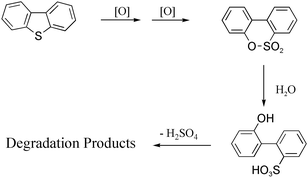
A catalytic system consisting of metal–sulfophthalocyanines (MPcS) and monopersulfate or hydrogen peroxide as oxidants was effective in the dibenzothiophene oxidative desulfurization with various yields and selectivities. Oxidations were conducted at room temperature in acetonitrile–water mixed solvent. The dibenzothiophene oxidation involved the step by step formation of dibenzothiophene dioxide and biphenylsultone (dibenzo-1,2-oxathiine 2,2-dioxide), followed by hydrolysis to 2(2′-hydroxybiphenyl)sulfonate and finally catalytic desulfurization to 2-hydroxybiphenyl (2-phenylphenol) and sulfuric acid; all the intermediate compounds were identified. Moreover, catalytic over-oxidation of 2-hydroxybiphenyl, with ring fission and formation of various oxidation products, among them carbon dioxide, oxalic and benzoic acid, was also observed. Among the various MPcS catalysts examined (M = Fe, Co and Ru), the ruthenium derivative exhibited the best performances with persulfate and iron derivative with hydrogen peroxide; in both cases the slow step of the process consisted in the oxidation of dibenzothiophene dioxide to biphenylsultone.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...