2∶1 Mg∶Al layered double hydroxide (LDH) with dodecylbenzene sulfonate in the gallery has been successfully prepared by the anion exchange and direct precipitation methods, and characterized by XRD, FTIR, SEM, EDX and AA. The interlayer spacing is 3.05 nm, which is in good agreement with the value estimated from an anti-parallel packing model with interpenetrating chains. We observe a high affinity of this surfactant anion for LDH in anion exchange and competition, exceeding that of sulfate and comparable to that of carbonate, and attribute this to hydrophobic interactions. More interestingly, the organic–inorganic self-assembled aggregates exhibit novel morphologies. These include bar-like particles that are several micrometers in length and tens to hundreds of nanometers in diameter, and curved as well as the more usual platy sheets. Contact with dodecylbenzene sulfonate solution places a hydrophobic surface on the normally hydrophilic Mg2Al(OH)6·0.5CO32−–LDH, allowing for miscible blending of inorganic and organic components in composites. Dodecylbenzene sulfonate is taken up in preference to chloride or, surprisingly, sulfate, and partly displaces even carbonate on refluxing.
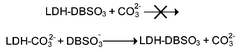
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
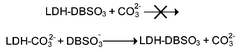

 Please wait while we load your content...
Please wait while we load your content...